The safety of Genix products and health and well-being of patients are highest priority of Genix. In order to provide the best possible information about our products to prescribers, patients and consumers, we must fully understand our products safety and quality profile.
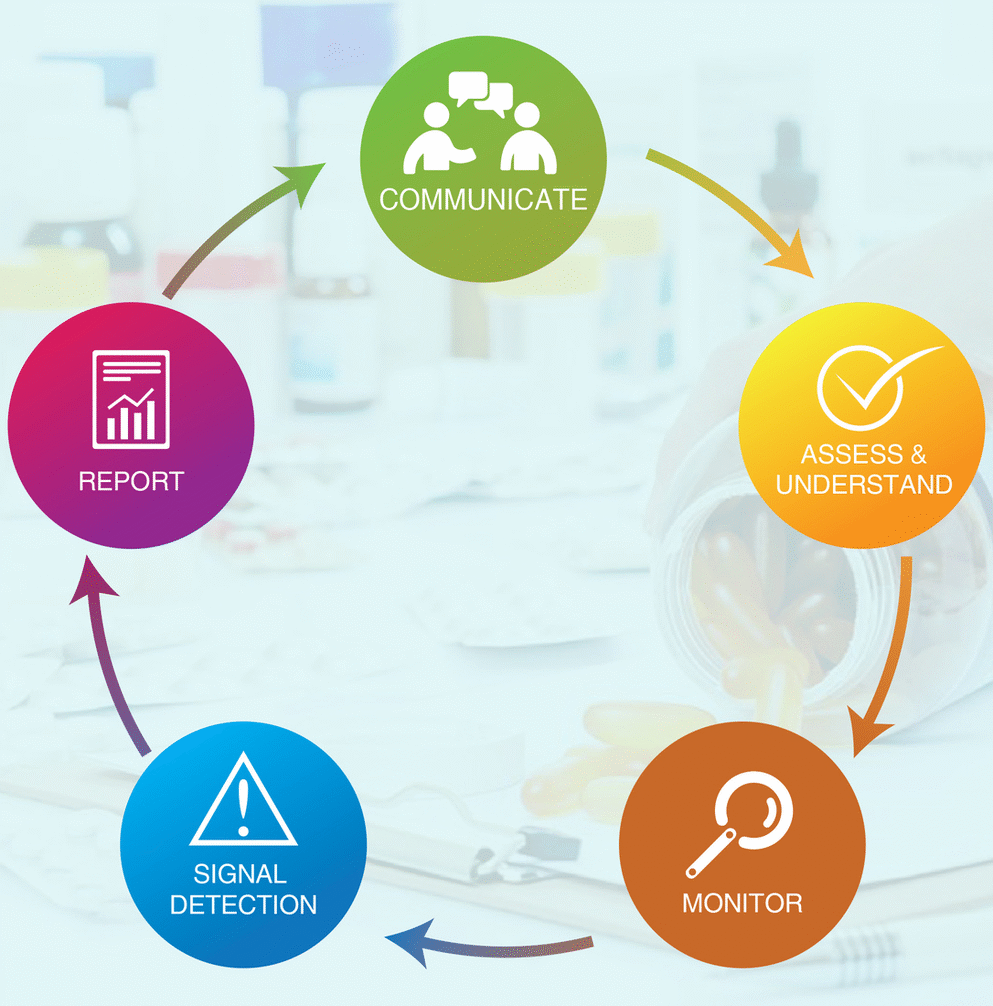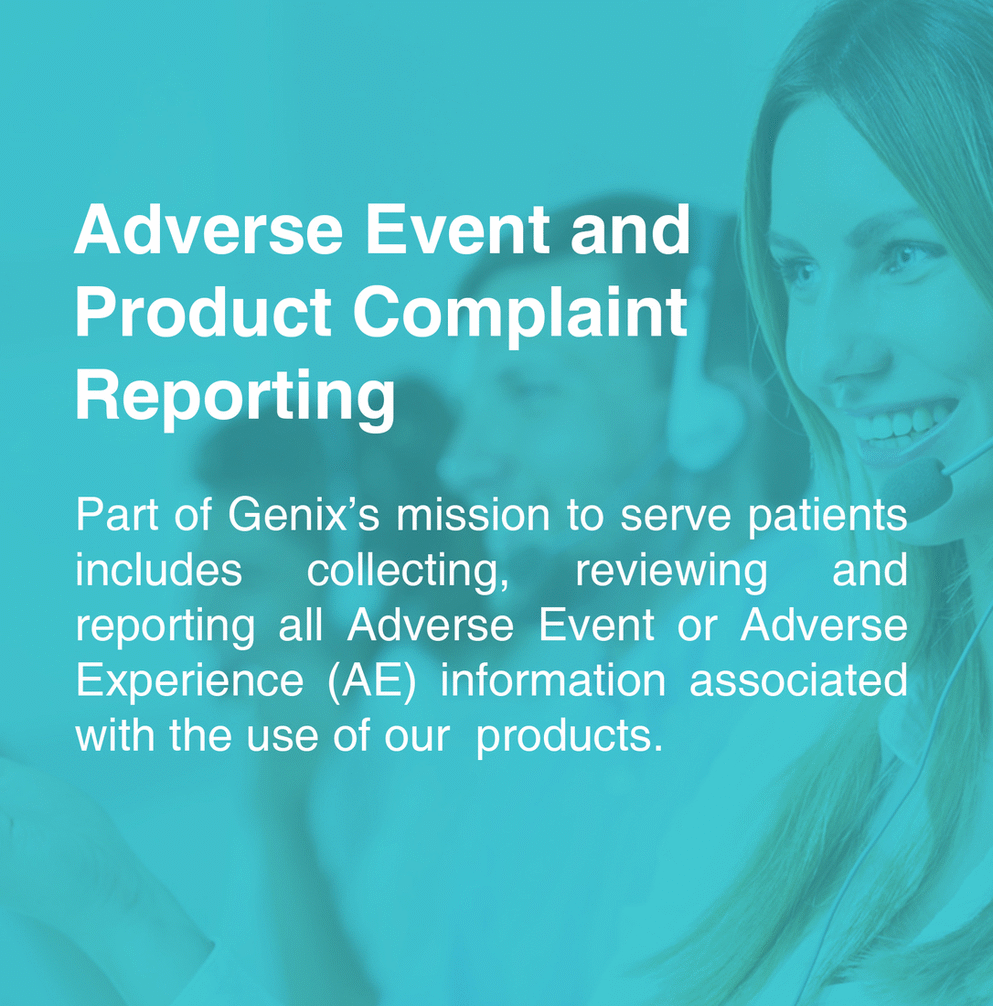
Genix is responsible for collecting, processing and evaluating the information relevant to the benefit-risk profile of its product. Any significant safety issues that may arise are promptly communicated to healthcare professionals, patients and regulatory authorities concerned in accordance with current regulations.
Spontaneous reporting of suspected adverse reactions is an important source of information for pharmacovigilance activities in order to correctly assess the safety, quality and performance of our products. In fact, we all share this important responsibility, even if it is not part of our usual job function. In case you come across any adverse events related to our products, please feel free to connect with Genix ’s Pharmacovigilance (PV) team.
In case you come across any adverse events related to our products, please feel free to connect with Genix ’s Pharmacovigilance (PV) team.
Phone :+92-21-111-10-10-11
Email Address :[email protected]
Privacy Policy: Genix respects your right of privacy. On our website we take the protection of personal data very seriously.